KANEKA KanCapA™ 3G represents a new cellulose-based protein A resin with a high dynamic binding capacity and an outstanding impurity removal characteristics due to its newly engineered protein A ligand.
KANEKA KanCapA™ 3G shows enhanced binding capacity, mainly at longer residence times, which makes it suitable for monoclonal antibody purification from high titer feedstocks.
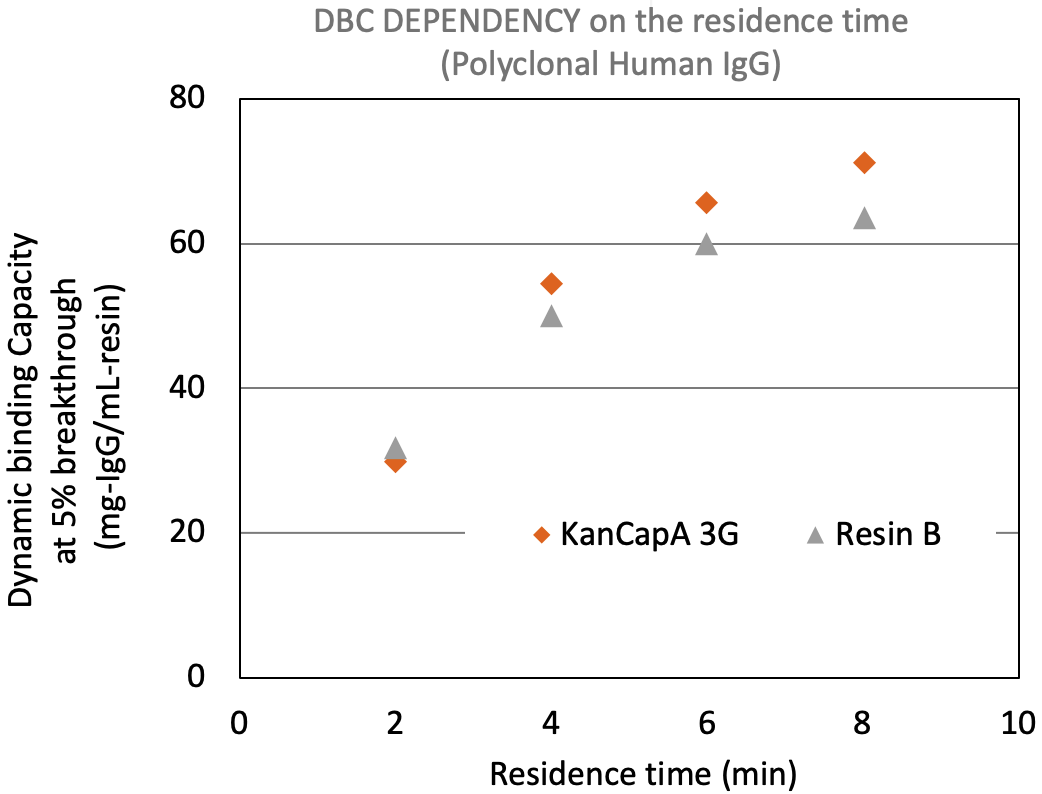
Residence time vs DBC (Polyclonal Human IgG)
KANEKA KanCapA™ 3Gʼs ligand was designed for efficient elution of monoclonal antibodies and their Fc fusion derivatives under mild acidic conditions.
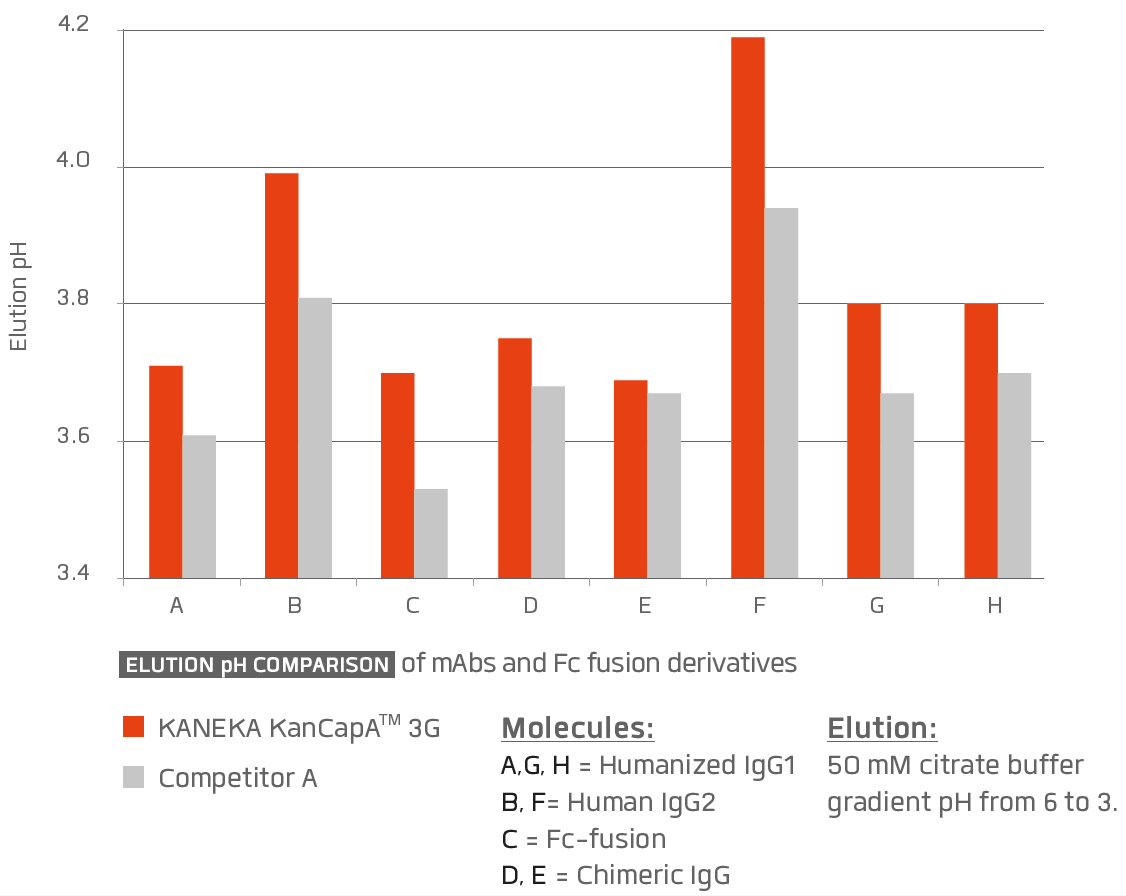
Comparison of the elution pH of KANEKA KanCapA™ 3G to competitor A. Elution pH was determined by a linear pH gradient elution using citrate buffer from pH 6 to pH 3.
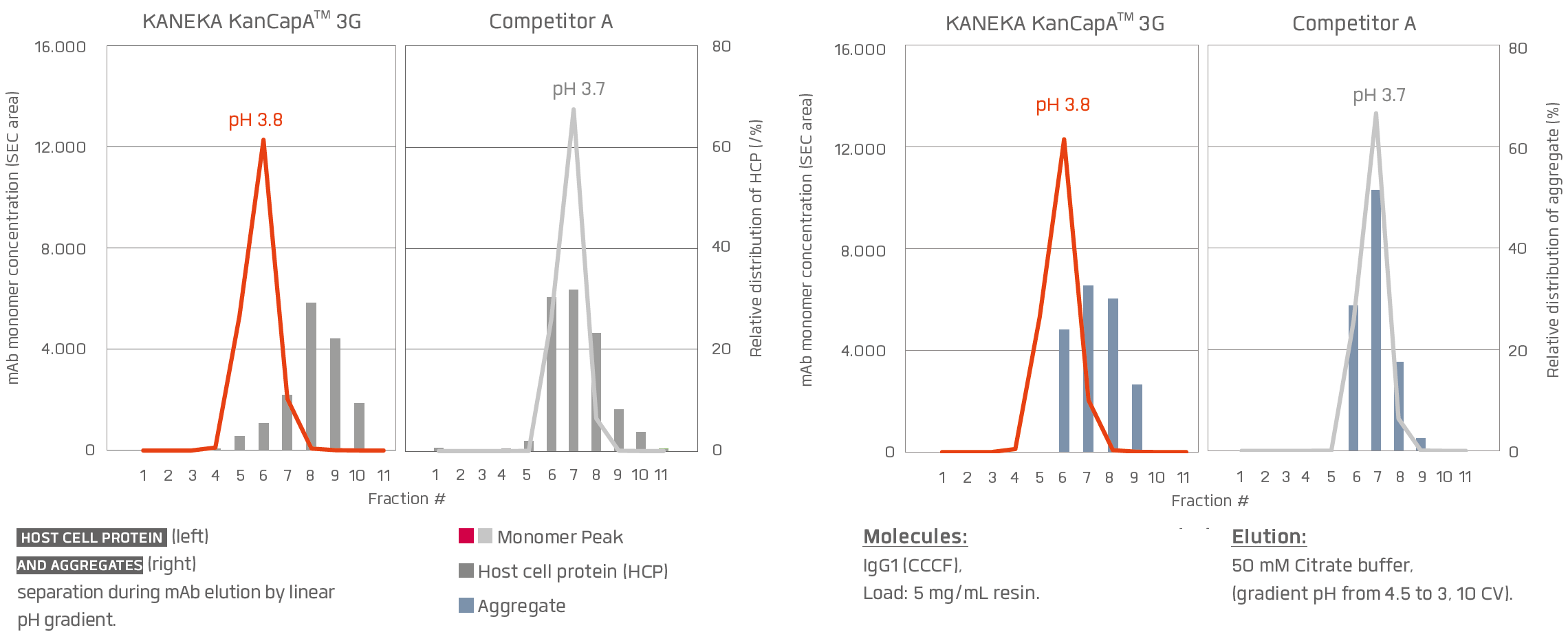
KANEKA KanCapA™ 3G has a better potential for impurity removal compared to current resins available on the market.
KANEKA KanCapA™ 3G is available in various formats adapted to all stages of the purification process from method optimization and process development to commercial manufacturing.
| Format | Volume | Catalog Number |
|---|---|---|
| Prepacked Column | 1mL | KPA03-C001 |
| 5mL | KPA03-C005 | |
| RoboColumn® | 200µL x 8 row | KPA03-S600 |
| 600µL x 8 row | KPA03-S600 | |
| Bulk Resin | 25 mL | KPA03-B025 |
| 500 mL | KPA03-B500 | |
| 5 L | KPA03-B05K | |
| 10 L | KPA03-B10K |