KANEKA KanCapA™ is a alkaline stable protein A affinity resin suitable for industrial scale purification of monoclonal antibodies. The Protein A ligand is a C-domain based pentamer that exhibits high alkaline stability and negligible Fab binding.
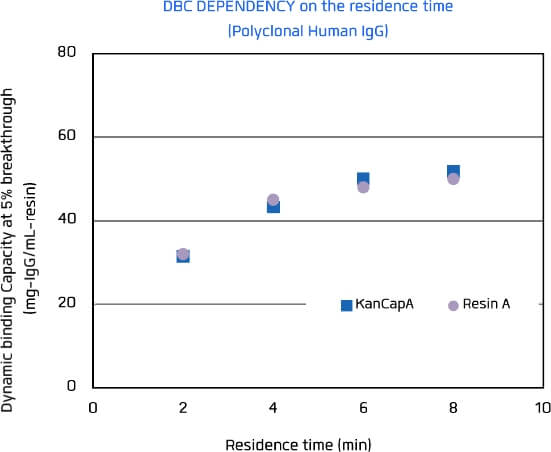
KANEKA KanCapA™ has been designed for efficient antibody capture achieving a high binding capacity at 3 to 6 minutes residence time.
KANEKA KanCapA™ also has the data compatible with major agarose protein A resin.
KANEKA KanCapA™’s ligand exhibits resistance to sodium hydroxide cleaning. The resin can be safely used up to 300 cycles with 0.1 M sodium hydroxide as the CIP solution with 15 minutes of contact time maintaining its binding capacity.
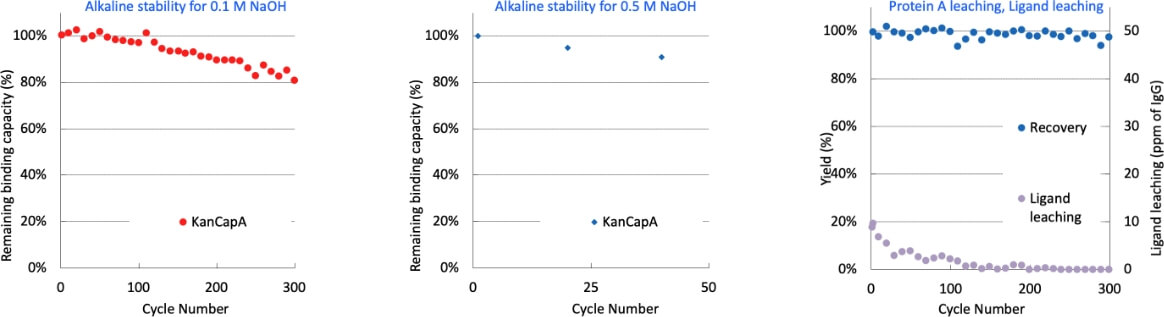
Life cycle study: Remaining dynamic binding capacity at 5 % breakthrough (RT : 3 min.) after repeated alkaline CIP with 0.1 M or 0.5 M NaOH (left); Yield and Protein A ligand leaching in the cycle use study with 0.1 M NaOH (right). Contact time to NaOH in all experiments is 15 minutes. Ligand leaching less than the detection limit (1 ppm) is plotted as 1 ppm.
KANEKA KanCapA™’s ligand binding ability to Fab region was eliminated in order to use mild acidic conditions for monoclonal antibody elution.
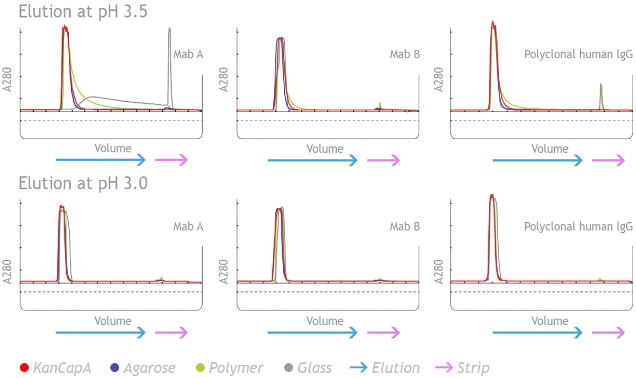
KANEKA KanCapA™ improves the elution profile of the VH3-encoded mAb purification compared to other commercially available Protein A resins. 5 mg/mL-resin of IgG are loaded and elution is performed at pH 3.5 and 3.0. Strip solution is 1 M Acetic acid.
Due to its matrix KANEKA KanCapA™ resin is perfectly suited for pilot and large scale purification processes. It can be efficiently packed into columns of various sizes either by normal flow or by axial compression methods. Packed columns show excellent pressure/flow rate characteristics and excellent performance. We have experience in packing up to 180 cm I.D.
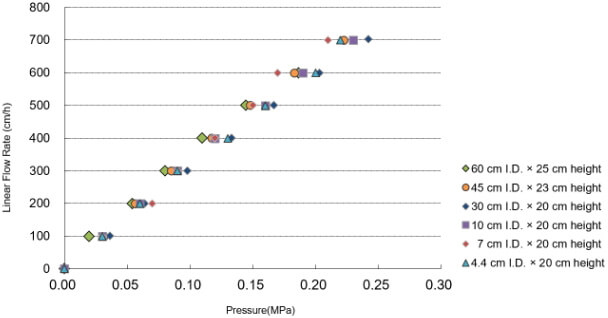
LPressure/flow rate characteristics of KANEKA KanCapA™ packed into various column sizes:
The pressure/ flow rate characteristics and packing efficiency of packed columns, from 4.4 cm to 60 cm I.D. and 20 cm to 25 cm bed height.
The pressures generated by packed beds are calculated by subtracting the system pressure from total pressure.
KANEKA KanCapA™ is available in various formats adapted to all stages of the purification process from method optimization and process development to commercial manufacturing.
| Format | Volume | Catalog Number |
|---|---|---|
| Prepacked Column | 1mL | KPA02-C001 |
| 5mL | KPA02-C005 | |
| RoboColumn® | 200µL x 8 row | KPA02-S200 |
| 600µL x 8 row | KPA02-S600 | |
| Bulk Resin | 25 mL | KPA02-B025 |
| 500 mL | KPA02-B500 | |
| 5 L | KPA02-B05K | |
| 10 L | KPA02-B10K |